Lithotripsy represents a cornerstone in modern urology and minimally invasive medicine, offering a targeted approach to disintegrate calculi (stones) within the body without the need for extensive surgery. Primarily used for kidney stones (nephrolithiasis), it also applies to ureteral, biliary, and salivary stones. This procedure leverages physical energy sources—such as shock waves, lasers, or ultrasound—to fragment stones into passable particles, alleviating symptoms like excruciating pain, hematuria (blood in urine), and potential renal damage from obstruction. With success rates often exceeding 80% for suitable cases, lithotripsy has transformed stone management from a surgical necessity to an outpatient procedure, reducing recovery times and complications.
The genesis of lithotripsy dates to the late 1970s in Munich, Germany, where researchers at the Dornier company, inspired by underwater explosive shock waves used in mining, pioneered extracorporeal shock wave lithotripsy (ESWL). The first animal trials in 1979 demonstrated stone fragmentation without tissue damage, leading to the inaugural human treatment in 1980 on a patient with a 1.5 cm renal stone. By 1983, the Dornier HM3 lithotripter—the first commercial device—received European approval, and in 1985, the U.S. Food and Drug Administration (FDA) cleared it for clinical use.
Early ESWL relied on electrohydraulic spark-gap generators, producing shock waves in a water bath for optimal transmission. This sparked a global surge in adoption; by the 1990s, over 80% of kidney stones in developed nations were treated non-invasively. Technological refinements followed: electromagnetic lithotripters (e.g., Siemens Lithostar) eliminated water baths for dry-table treatments, improving patient comfort. Piezoelectric systems (e.g., Wolf Piezolith) used arrays of crystals for focused, low-energy waves, minimizing pain.
The 21st century brought integration with advanced imaging—fluoroscopy, ultrasound, and computed tomography (CT)—for real-time stone localization. Dual-energy CT now differentiates stone composition pre-procedure, predicting fragmentation efficacy. Burst-wave lithotripsy (BWL), an emerging technique using rapid micro-shock waves, shows promise in preclinical studies for faster, less painful treatments. Globally, over 1 million ESWL sessions occur annually, with adaptations for gallstones (via endoscopic approaches) and even pancreatic stones.
To appreciate lithotripsy’s role, understanding stone etiology is essential. Urolithiasis affects 8-10% of the population, with higher incidence in men (2:1 ratio) and peaks at ages 30-50. Stones form via supersaturation of urine with lithogenic substances, influenced by diet, genetics, metabolism, and environment. Dehydration concentrates urine, promoting nucleation and crystal growth in the renal tubules, pelvis, or calyces.
Key stone types, classified by composition (determined via infrared spectroscopy or X-ray diffraction post-retrieval), dictate lithotripsy suitability:
Calcium-Based Stones (75-85%): Predominantly calcium oxalate (monohydrate or dihydrate) or calcium phosphate (apatite or brushite). Oxalate stones, often from hyperoxaluria (e.g., spinach-rich diets or enteric disorders like Crohn’s), are dense (Hounsfield units >1000 on CT) and ESWL-resistant if >1 cm. Phosphate stones link to alkaline urine from renal tubular acidosis or hyperparathyroidism; they fragment more readily.
Struvite Stones (10-15%): Composed of magnesium ammonium phosphate, these “infection stones” arise from urease-producing bacteria (e.g., Proteus, Klebsiella) in chronic UTIs. They form staghorn calculi branching into renal calyces, obstructing drainage and risking pyelonephritis. Lithotripsy succeeds if infection is cleared first, but recurrence is high without antibiotics.
Uric Acid Stones (5-10%): Radiolucent on plain X-rays but visible on CT/ultrasound, these form in acidic urine (pH <5.5) from hyperuricosuria (gout, chemotherapy, or purine-heavy diets). They dissolve with urinary alkalinization (potassium citrate), making pre-lithotripsy chemolysis viable; ESWL efficacy is moderate due to fragility.
Cystine Stones (1-2%): Hereditary (cystinuria gene mutation), these hexagonal crystals resist all lithotripsy modalities due to hardness. Combined therapies (e.g., ESWL + tiopronin chelation) are needed.
Rare Types: Xanthine (from allopurinol) or drug-induced (indinavir) stones require tailored approaches.
Obstruction leads to hydronephrosis, impairing glomerular filtration and risking acute kidney injury. Chronic cases promote hypertension or end-stage renal disease. Lithotripsy interrupts this cascade by enabling fragment passage, restoring flow.
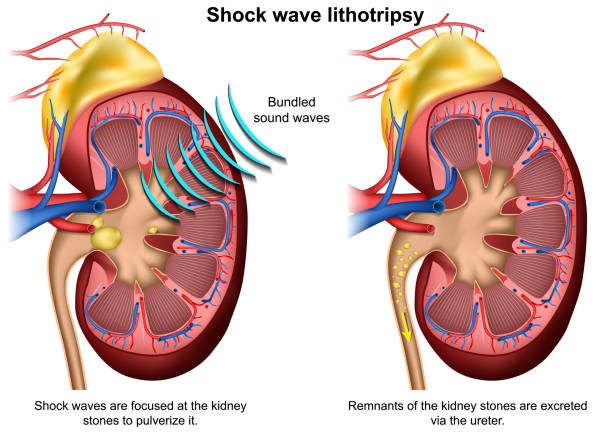
The gold standard for noninvasive treatment, ESWL targets stones 4-20 mm in the kidney or proximal/mid-ureter. Shock waves (pressure peaks >50 MPa, propagating at 1500 m/s) originate extracorporeally, focusing via ellipsoidal reflectors (F2 focal point on the stone). Energy dissipates as cavitation bubbles implode, generating shear forces that crack the stone.
Procedure Details: Performed under sedation or analgesia (e.g., fentanyl). Patient positioning: prone/supine on a lithotripter (e.g., Storz Modulith). Imaging localizes the stone; a coupling gel or water cushion transmits waves. Sessions last 45-90 minutes, delivering 2000-4000 shocks at 60-120/min, starting at 14-18 kV and ramping to 20-22 kV. Voltage is titrated to avoid pain (Steinstraße risk from clustered fragments).
Efficacy: 70-90% stone-free rate (SFR) at 3 months for <10 mm stones; drops to 50% for >20 mm. Repeat sessions (up to 3) boost success.
Advantages: No anesthesia, outpatient (discharge same day), low cost (~$5000 USD).
For stones inaccessible to ESWL (e.g., distal ureter, impacted calculi), energy is delivered endoscopically.
Laser Lithotripsy: Holmium:YAG (Ho:YAG) lasers (2100 nm wavelength) via ureteroscope or nephroscope vaporize stones with minimal retropulsion. Fiber diameters (200-365 μm) allow precise dusting or fragmentation. Success: 95% for ureteral stones; used in flexible ureterorenoscopy (fURS) for lower pole kidneys.
Ultrasonic Lithotripsy: Pneumatic or ultrasonic probes (20-30 kHz) in rigid nephroscopes shatter stones via mechanical vibration. Effective for larger stones during percutaneous nephrolithotomy (PCNL), with basket extraction of fragments.
Electrohydraulic Lithotripsy (EHL): Spark-generated underwater shocks via ureteroscope; high efficacy but risks mucosal injury, limiting use.
Pre-procedure assessment is multifaceted:
Imaging: Non-contrast CT (gold standard) measures size, density (HU), and skin-to-stone distance (SSD >10 cm reduces efficacy). Ultrasound detects hydronephrosis; IV pyelogram (IVP) assesses anatomy.
Labs: Urinalysis/culture (rule out UTI), serum creatinine (renal function), coagulation profile (PT/PTT), and stone risk panel (24-hour urine for calcium, oxalate, citrate, uric acid).
Contraindications: Absolute—pregnancy (teratogenic shock waves), aortic aneurysm, untreated coagulopathy. Relative—obesity (BMI >35), distal obstructions, hard stones (brushite/cystine).
Preparation: Hydration (2-3 L/day), alpha-blockers (tamsulosin 0.4 mg) for ureteral relaxation, and antibiotics if infected. Fasting 6-8 hours if sedated.
During ESWL, vital signs are monitored; ECG for cardiac synchronization avoids arrhythmias. Pain is scored via VAS; opioids or paracervical blocks mitigate it.
Post-procedure: Observe 1-2 hours for hypotension or gross hematuria. Discharge criteria: stable vitals, voiding normally. Instructions: Strain urine (for fragment analysis), hydrate aggressively, avoid NSAIDs initially (renal risk). Alpha-blockers continue 4-6 weeks to facilitate expulsion (passage rate 80% for <4 mm fragments).
Follow-up: KUB X-ray/ultrasound at 1-2 weeks; CT at 3 months for SFR. If residual fragments >4 mm, intervene.
Complications occur in 10-20% of cases, mostly minor:
Mitigation: Low shock rates, imaging guidance, prophylactic antibiotics. Radiation exposure: 1-3 mSv per session (comparable to background yearly).
In the landscape of modern medicine, lithotripsy stands as a testament to innovative, patient-centered care, fundamentally altering the treatment paradigm for urolithiasis and related calculi. From its groundbreaking inception in the 1980s with extracorporeal shock wave lithotripsy (ESWL) to today’s sophisticated intracorporeal laser and ultrasonic modalities, this procedure has democratized stone management, shifting from invasive surgeries to efficient, minimally disruptive interventions. By harnessing focused energy to fragment stones—whether calcium oxalate behemoths in the kidney or uric acid obstructions in the ureter—lithotripsy achieves stone-free rates of 70-95% in appropriately selected cases, while minimizing risks like infection, bleeding, and prolonged recovery. Its outpatient feasibility, cost-effectiveness, and integration with advanced imaging (e.g., CT-guided targeting) have reduced global healthcare burdens, sparing millions from the morbidity of open procedures and enabling same-day returns to daily life.
Chat With Me