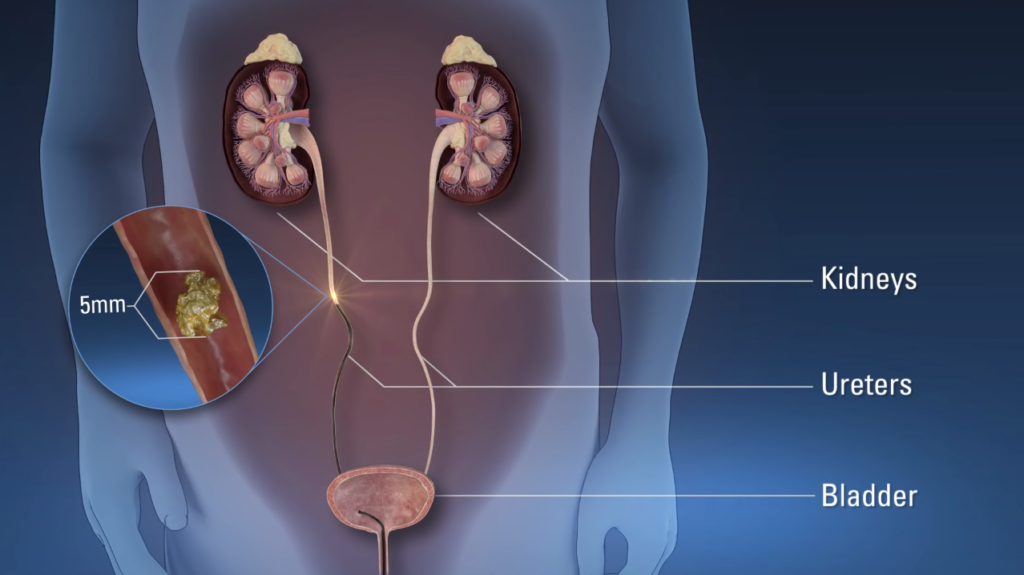
Ureteroscopy is a cornerstone procedure in modern urology, representing a minimally invasive endoscopic technique designed to visualize, diagnose, and treat pathologies within the upper urinary tract. This includes the ureters (the tubes connecting the kidneys to the bladder), the renal pelvis, and the intrarenal collecting system (calyces). The procedure utilizes a ureteroscope—a slender, fiberoptic or digital instrument equipped with a light source, camera, and working channels for instruments—to access these areas endoscopically via a natural route: the urethra and bladder. First introduced in the 1970s by pioneers like Lyon and Goodman, ureteroscopy has evolved from a rudimentary diagnostic tool into a versatile therapeutic modality, largely due to advancements in optics, miniaturization, and energy sources like lasers.
Unlike open surgery or percutaneous approaches, ureteroscopy avoids external incisions, reducing morbidity, hospital stays, and recovery times. It is performed under general or spinal anesthesia in an operating room, typically lasting 30-120 minutes, and is outpatient or short-stay in most cases. Success rates for stone management exceed 85-95%, depending on stone characteristics, making it a first-line intervention for many urolithiasis cases.
Ureteroscopy’s indications span diagnostic and therapeutic realms, primarily driven by the need to address obstructive or symptomatic conditions in the upper urinary tract.
1. Urolithiasis (Kidney and Ureteral Stones): The most common indication, accounting for over 80% of procedures. Ureteroscopy is ideal for stones in the ureter (ureteral calculi) or kidney (nephrolithiasis), particularly those 5-20 mm in size. Distal ureteral stones (below the pelvic brim) are easily accessible with rigid scopes, while proximal or intrarenal stones require flexible scopes. It is preferred when stones are in unfavorable locations for extracorporeal shock wave lithotripsy (ESWL), such as impacted stones, those in narrow ureters, or in patients with anatomical variants like horseshoe kidneys. Stone composition influences choice: calcium-based stones (oxalate or phosphate) fragment well with lasers, while uric acid or cystine stones may require chemolysis adjuncts.
2. Upper Tract Urothelial Carcinoma (UTUC): Ureteroscopy enables direct visualization and biopsy of suspicious lesions in the ureter or renal pelvis, crucial for staging and management of this rare cancer (incidence ~1-2 per 100,000). Techniques like fluorescence in situ hybridization (FISH) or narrow-band imaging enhance detection of flat, non-papillary tumors. For low-grade, low-stage disease, endoscopic ablation with laser or electrocautery can be curative, avoiding nephroureterectomy.
3. Ureteral Strictures and Obstructions: Narrowing due to iatrogenic causes (e.g., prior ESWL or surgery), inflammation (e.g., endometriosis, tuberculosis), or extrinsic compression (e.g., tumors, retroperitoneal fibrosis). Ureteroscopy allows balloon dilation (using 4-12 Fr balloons inflated to 8-20 atm for 5-10 minutes) or incision (endoureterotomy) under direct vision, with success rates of 50-80% for short strictures (<1 cm).
4. Hematuria Evaluation: For persistent or gross hematuria of upper tract origin, ureteroscopy identifies sources like vascular malformations, polyps, or clots, guiding targeted therapy.
5. Foreign Bodies and Migrated Stents: Retrieval of retained fragments, coils, or malpositioned stents that cause obstruction or infection.
6. Diagnostic Exploration: In cases of unexplained flank pain, hydronephrosis, or positive cytology without bladder findings, to rule out occult pathology.
Contraindications include active untreated urinary tract infections (to prevent sepsis), uncorrectable coagulopathies (e.g., INR >1.5), and severe ureteral tortuosity precluding access. Relative contraindications encompass pregnancy (due to fluoroscopy radiation) and solitary kidneys, where preservation is paramount.
Ureteroscopes are classified by rigidity, diameter, and deflection capabilities, each suited to specific anatomical segments.
Rigid Ureteroscopes: Straight, metal instruments (6-12 Fr outer diameter) with high-resolution optics. They excel in the distal two-thirds of the ureter due to their rigidity, providing stable visualization and efficient irrigation. Examples include the Karl Storz or Olympus models. Advantages: Superior image quality, larger working channels (up to 5 Fr) for baskets/forceps, and lower cost. Disadvantages: Limited to straight paths; cannot access the proximal ureter or kidney without risk of perforation. Used primarily for distal stones.
Flexible Ureteroscopes: Semi-rigid or fully flexible, with tip deflection up to 270-360 degrees (active and passive). Diameters range from 7.5-9.8 Fr, with working channels of 3.6-4.5 Fr. Digital versions (e.g., Boston Scientific LithoVue) use CMOS chips for chip-on-tip imaging, offering HD video and integrated instrument tracking. Fiberoptic models (e.g., Olympus URF-V) rely on fiber bundles but are more durable. Advantages: Access to the entire collecting system, including upper pole calyces; versatility for complex cases. Disadvantages: Fragility (durability ~20-50 uses), reduced irrigation flow, and higher cost (~$20,000-50,000 per scope). Single-use disposable scopes (e.g., LithoVue Elite) mitigate infection risks and cross-contamination.
Semi-Rigid Ureteroscopes: Hybrid designs for mid-ureter access, bridging rigid and flexible capabilities.
Selection depends on pathology: rigid for simple distal work, flexible for intrarenal or proximal disease. Ureteral access sheaths (UAS, 9.5-12 Fr) are often used with flexible scopes to facilitate re-entry, improve outflow, and reduce pressure (maintaining <40 mmHg to avoid pyelovenous backflow).
Thorough preparation optimizes outcomes and minimizes risks.
Evaluation: History and physical exam assess symptoms (e.g., colic pain, hematuria), comorbidities (e.g., diabetes increasing infection risk), and prior interventions. Urinalysis screens for infection (leukocytes, nitrites); culture guides antibiotics if positive. Blood work includes CBC, coagulation profile, renal function (creatinine, eGFR), and electrolytes.
Imaging: Non-contrast CT urography is gold standard, providing stone size, location, density (Hounsfield units >1000 predict laser efficacy), and anatomy (e.g., ureteral diameter <7 mm may need dilation). Alternatives: Ultrasound for radiation avoidance, IVP for functional assessment, or MRI in pregnancy.
Medical Optimization: Discontinue antiplatelets (aspirin 7 days prior) or anticoagulants (bridge with heparin if high-risk). Alpha-1 blockers (tamsulosin 0.4 mg daily for 1-2 weeks) relax ureteral orifices, easing access (success rate boost ~20%). Low-dose aspirin may continue in cardiac patients. Antibiotic prophylaxis (e.g., cefazolin 2g IV) per AUA guidelines, especially if stones >10 mm or stents planned.
Patient Counseling: Discuss anesthesia risks, procedure details, stent discomfort (80% experience), and alternatives. Informed consent covers success rates (85-95% stone-free), complications (5-10%), and recovery (1-3 days off work).
Bowel Prep and Fasting: Optional laxatives for obese patients; NPO 6-8 hours pre-op.
Ureteroscopy is performed in the lithotomy position under general anesthesia for optimal relaxation.
Cystoscopy and Access: A 16-22 Fr cystoscope visualizes the bladder and ureteral orifices. A 0.038-inch safety guidewire (e.g., Teflon-coated) is advanced under fluoroscopy into the ureter, ideally to the kidney. A second working wire may be placed. If orifice is tight, balloon dilation (4-6 Fr, 2-4 atm) or incision precedes.
Ureteroscope Insertion: The scope advances over the wire. Rigid scopes go first for distal pathology; flexible for proximal. Saline irrigation (room temperature or warmed to 37°C) maintains clarity, pressure <100 mmHg via gravity or hand-pump. Fluoroscopy confirms position.
Visualization and Intervention:
Completion: Residual fragments flushed or basketted. UAS removed if used. A double-J stent (5-6 Fr, 24-28 cm) is placed over wire to bridge ureteropelvic junction to bladder, preventing edema/obstruction. Cystoscopy confirms positioning.
Intraoperative challenges include “stone rain” (fragment migration), managed by UAS or lower power settings. Monitoring includes vital signs, end-tidal CO2, and intrarenal pressure via manometry.
Immediate Post-Op: PACU monitoring for 1-2 hours. Pain control with IV ketorolac/acetaminophen; opioids if needed. Urinalysis checks hematuria; antibiotics continued 24 hours.
Stent Management: Indwelling 1-14 days (shorter for uncomplicated cases). Symptoms (flank pain, urgency, hematuria) peak days 2-4; alpha-blockers (tamsulosin) and PPIs reduce. Removal via string pull or office cystoscopy.
Discharge and Follow-Up: Same-day or 23-hour observation. Instructions: Hydrate (2-3L/day), strain urine for fragments, avoid heavy lifting 1 week. Follow-up at 1-2 weeks: Imaging (KUB/X-ray or low-dose CT) assesses stone-free rate (SFR; <2 mm fragments acceptable). Metabolic workup (24-hour urine) for recurrent stone formers: Target volume >2.5L, citrate >320 mg/day, etc.
Recovery Timeline: Mild dysuria/hematuria 3-7 days; full activity in 1-2 weeks. Complications like sepsis (1-2%) require readmission.
Complications occur in 5-15%, mostly minor (Clavien I-II).
Risk factors: Large stones, long procedures (>90 min), no UAS. AUA guidelines emphasize technique refinement to minimize.
Technological leaps have transformed ureteroscopy. Digital scopes provide 4K imaging and deflect ~270° bidirectionally. Robotic platforms (e.g., Avicenna Roboflex) enable surgeon-controlled arms for precision, reducing fatigue in long cases. AI integration analyzes endoscopy feeds for automatic stone detection or composition via Raman spectroscopy, optimizing laser parameters.
Disposable scopes curb sterilization costs and infections (e.g., CRE outbreaks). Thulium fiber lasers (TFL) fragment faster with less fiber degradation. Micro-optics allow pediatric use (4.5 Fr scopes).
Research focuses on outpatient stenting avoidance via alpha-blockers or novel coatings. Gene therapy for stone prevention and nanoparticle-targeted lithotripsy are emerging.
Comparisons:
In the ever-evolving landscape of urological practice, ureteroscopy stands as a testament to the power of minimally invasive innovation, transforming the management of upper urinary tract disorders from high-risk open surgeries to precise, patient-centered interventions. This procedure, which began as a diagnostic curiosity in the mid-20th century, has matured into a gold-standard therapy for urolithiasis, urothelial carcinoma, strictures, and beyond, boasting stone-free rates of 85-95% and complication profiles that pale in comparison to alternatives like percutaneous nephrolithotomy or laparoscopy. By leveraging advanced ureteroscopes—rigid for straightforward distal access and flexible/digital for intricate intrarenal navigation—clinicians can visualize, biopsy, and treat pathologies with unprecedented accuracy, all while minimizing tissue trauma, radiation exposure, and recovery downtime. The integration of tools like the Ho:YAG or thulium fiber lasers, ureteral access sheaths, and high-definition optics has not only elevated success but also democratized care, making it feasible for diverse populations, from pediatrics to the elderly, and in resource-constrained settings.
Chat With Me