Renal biopsy, also known as kidney biopsy, is a critical diagnostic procedure in nephrology that involves the extraction of a small sample of kidney tissue for microscopic examination. This invasive yet invaluable tool allows clinicians to identify the underlying causes of kidney dysfunction, guiding precise treatment strategies. By providing direct insight into the renal parenchyma, it surpasses the limitations of non-invasive tests like blood work, urinalysis, or imaging, which often offer only indirect clues. First introduced in the 1950s with the advent of percutaneous techniques, renal biopsy has evolved into a cornerstone of modern kidney medicine, performed annually on hundreds of thousands of patients worldwide. Its role is particularly vital in an era of rising chronic kidney disease (CKD) prevalence, affecting over 500 million people globally, according to the World Health Organization.
The primary purpose of a renal biopsy is to diagnose and characterize renal pathologies. Kidneys filter waste from the blood, regulate electrolytes, and maintain fluid balance, but diseases like glomerulonephritis, diabetic nephropathy, or interstitial nephritis can impair these functions subtly at first. Symptoms such as proteinuria (excess protein in urine), hematuria (blood in urine), unexplained acute kidney injury (AKI), or rapidly progressive renal failure often prompt a biopsy. For instance, in nephrotic syndrome—characterized by heavy proteinuria, edema, and hypoalbuminemia—a biopsy can differentiate between minimal change disease (which responds well to steroids) and focal segmental glomerulosclerosis (FSGS), which may require immunosuppression or even transplantation evaluation. In transplant recipients, biopsies monitor for rejection or infection, with protocols like the Banff classification standardizing interpretations.
Indications for renal biopsy are carefully weighed against risks, typically reserved for cases where diagnosis impacts management. Common scenarios include unexplained nephrotic or nephritic syndromes, isolated hematuria or proteinuria in adults or children, systemic diseases like lupus nephritis or vasculitis affecting the kidneys, and evaluation of kidney allograft dysfunction. It’s less indicated in clear-cut cases like advanced diabetic nephropathy with retinopathy, where biopsy might not alter therapy. Pediatric biopsies are common for congenital disorders, while in the elderly, it’s used cautiously due to higher complication rates.
Renal biopsies are classified into three main types: percutaneous, open (surgical), and transjugular. The percutaneous approach, accounting for over 90% of procedures, is the gold standard due to its minimally invasive nature. Performed under local anesthesia with ultrasound or CT guidance, a thin needle (usually 14-18 gauge) is inserted through the skin into the lower pole of the kidney—often the left one, as it’s more accessible and less prone to complications from the liver. The patient lies prone, and real-time imaging ensures the needle targets the renal cortex, avoiding major vessels. Spring-loaded automated biopsy guns, like the Bard Biopty, capture 1-3 cores of tissue (each 1-2 cm long) in a single pass or multiple passes for adequate sampling. The procedure lasts 20-30 minutes, with sedation if needed for anxious patients.
Open renal biopsy, though rarer, is employed when percutaneous access is infeasible—such as in solitary kidneys, severe obesity, bleeding disorders, or anatomical anomalies. Under general anesthesia, a surgeon makes a small flank incision to directly visualize and excise tissue, often yielding larger samples but with higher risks of infection and longer recovery. Transjugular biopsy, an emerging alternative, accesses the kidney via the internal jugular vein using a catheter, ideal for patients with coagulopathies or ascites. Fluoroscopy guides the process, minimizing bleeding risks since the venous approach tamponades naturally. This method, popularized in the 1990s, is particularly useful in liver-kidney syndromes or high-risk AKI cases.
Preparation for a renal biopsy is meticulous to optimize safety. Patients undergo baseline labs: complete blood count (CBC) to check platelet levels (>50,000/μL ideally), coagulation profile (PT/INR <1.5), and renal function tests. Those on anticoagulants like warfarin or antiplatelets (e.g., clopidogrel) must hold them for 5-7 days, with bridging therapy if high thrombotic risk. Hypertension is controlled (<140/90 mmHg), and informed consent details risks, including a 1-5% major complication rate. Fasting for 6-8 hours precedes the procedure, and prophylactic antibiotics are rare unless infection risk is elevated.
Post-procedure care focuses on hemorrhage prevention, the most common issue. Patients are monitored in a recovery unit for 4-6 hours, then bed rest for 12-24 hours with frequent vital signs checks. The biopsy site is compressed, and serial hemoglobin levels track for occult bleeding. Pain is managed with acetaminophen; NSAIDs are avoided. Gross hematuria occurs in 10-20% but resolves spontaneously, while perinephric hematoma (visible on ultrasound) affects up to 90% subclinically. Discharge criteria include stable vitals, no significant hematuria, and ability to void. Patients are advised to avoid heavy lifting for a week and report fever, severe flank pain, or persistent bleeding.
Complications, while infrequent, can be serious. Minor issues include pain, bruising, or transient hematuria. Major risks—occurring in 1-2%—encompass significant hemorrhage requiring transfusion (0.5-1%), arteriovenous fistula (AVF, 1%), or infection (0.1%). AVF may cause hypertension or bruit but often self-resolves; persistent cases need embolization. Rare events like pneumothorax (if the needle punctures the pleura) or organ injury occur in <0.1%. Mortality is exceedingly low (<0.1%), usually from uncontrolled bleeding in coagulopathic patients. Risk factors include small kidneys (<9 cm), hypertension, amyloidosis, or multiple passes. Advances like color Doppler ultrasound have reduced these by 50% over decades.
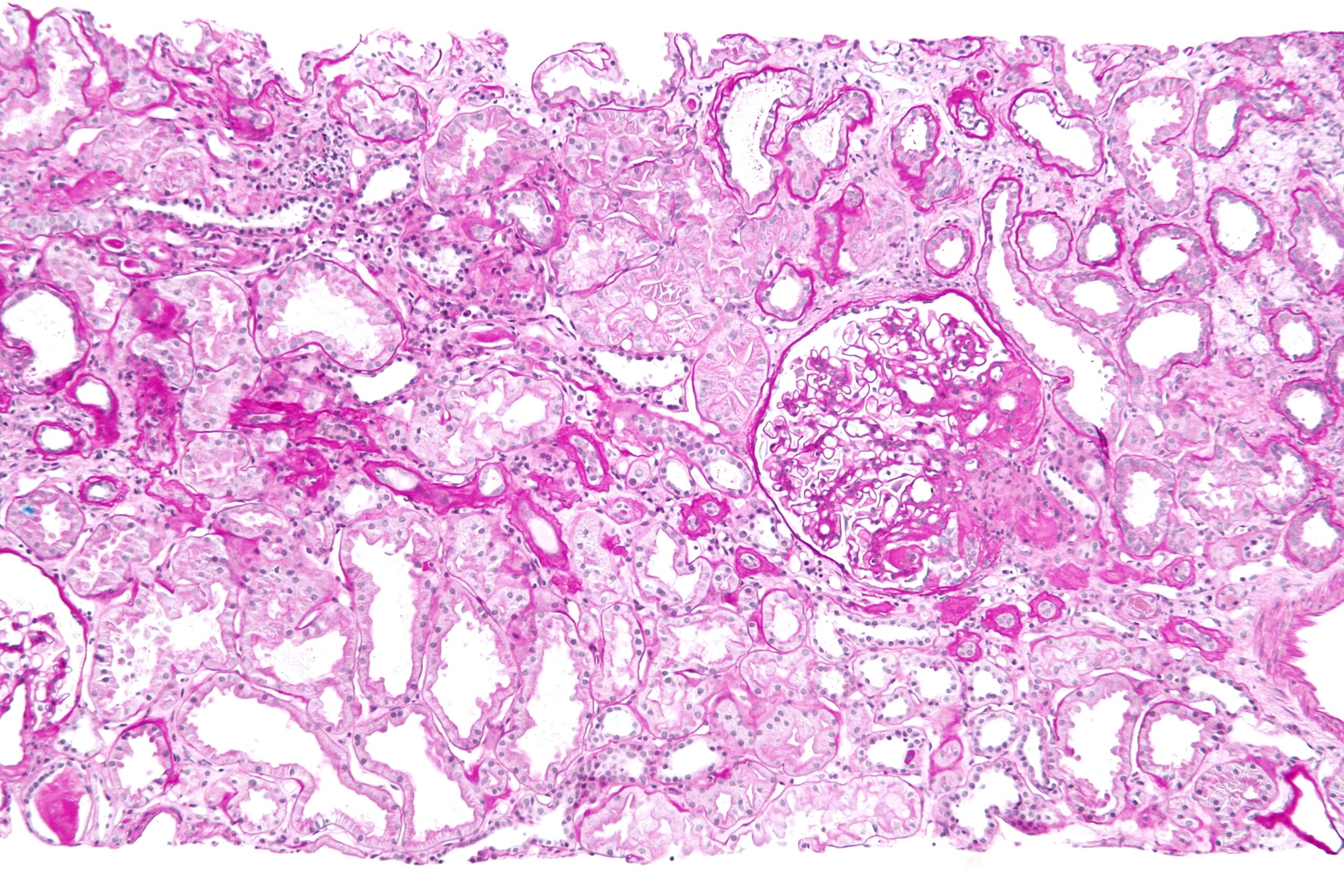
Once obtained, the biopsy sample is divided for multimodal analysis, yielding a definitive diagnosis in 95% of cases. Light microscopy (LM) examines paraffin-embedded sections stained with hematoxylin-eosin, periodic acid-Schiff (PAS), silver, and Masson’s trichrome, revealing glomerular, tubular, interstitial, and vascular changes. For example, it distinguishes proliferative from sclerosing glomerulopathies. Immunofluorescence (IF) on frozen sections detects immune deposits using antibodies against IgG, IgA, C3, etc., crucial for immune-mediated diseases like IgA nephropathy (Berger’s disease) or membranous nephropathy. Electron microscopy (EM) provides ultrastructural detail, identifying podocyte effacement in minimal change disease or subepithelial deposits in membranous glomerulopathy. Molecular techniques, like gene sequencing for hereditary nephropathies (e.g., Alport syndrome), are increasingly integrated.
Interpretation requires expertise from renal pathologists, correlating findings with clinical data. A typical report includes a semi-quantitative scoring system, such as the activity/chronicity index for lupus nephritis, influencing therapy. For instance, a biopsy showing crescentic glomerulonephritis might prompt urgent plasmapheresis and cyclophosphamide. In transplants, it detects antibody-mediated rejection via C4d staining. Limitations exist: sampling error (e.g., missing focal lesions) affects 5-10%, and artifacts from processing can mislead. Artificially intelligent tools are emerging to enhance accuracy.
Alternatives to biopsy include serological tests (e.g., anti-GBM antibodies for Goodpasture’s), genetic panels for inherited diseases, or advanced imaging like MRI elastography for fibrosis assessment. Non-invasive biomarkers, such as urinary mRNA or proteomic profiles, show promise but lack biopsy’s precision. In resource-limited settings, empirical treatment based on clinical patterns may suffice, but biopsy remains superior for atypical presentations.
Building upon the foundational overview of renal biopsy, this section delves deeper into its historical evolution, procedural nuances, disease-specific applications, ethical considerations, and emerging innovations. As a procedure that bridges clinical suspicion with histopathological truth, renal biopsy’s intricacies reveal why it remains indispensable despite non-invasive alternatives.
The origins of renal biopsy trace back to the early 20th century, but its modern form emerged post-World War II. In 1930, German pathologist Hermann Ribbert performed the first open kidney biopsy during autopsy, but clinical application lagged due to infection fears. The percutaneous era began in 1951 when Swedish nephrologist Nils Alwall introduced needle aspiration under local anesthesia, initially with crude Vim-Silverman needles that yielded fragmented samples. By 1958, American urologist John Kark refined the technique using the Franklin-Vim needle, establishing it as a routine outpatient procedure. The 1960s saw the integration of imaging—first intravenous pyelography, then ultrasound in the 1970s—reducing blind punctures and complications.
Key milestones include the 1980s adoption of automated biopsy guns, which improved core quality and safety, and the 1990s rise of transjugular biopsy by Jean-Paul Mroueh and colleagues, addressing high-risk patients. The Human Genome Project in the 2000s spurred molecular pathology, allowing biopsies to detect genetic mutations in real-time. Today, over 100,000 percutaneous biopsies occur annually in the U.S. alone, per the United States Renal Data System (USRDS), reflecting a 20-fold increase since the 1960s. This evolution mirrors nephrology’s shift from empirical to evidence-based practice, with biopsy-driven classifications like the Oxford MEST score for IgA nephropathy transforming prognostication.
While the percutaneous method dominates, variations cater to diverse patient needs. In ultrasound-guided biopsies, a high-frequency linear probe (3-5 MHz) visualizes the kidney in longitudinal view, with the needle trajectory marked by a sterile grid. The biopsy gun’s “pop” sound signals tissue capture; operators aim for 10-20 glomeruli per core for diagnostic adequacy, as fewer than seven may lead to underdiagnosis. For obese patients (BMI >35), CT guidance with contrast enhances precision, though radiation exposure is a trade-off.
In pediatric settings, biopsies are adapted for smaller kidneys (e.g., 6-8 cm in infants). General anesthesia is common, and finer needles (18-20 gauge) minimize trauma. The prone position is challenging for young children, so lateral decubitus or supine approaches with fluoroscopy are used. Success rates exceed 95%, but complications like hematoma rise to 5% due to immature coagulation. Protocols from the International Pediatric Nephrology Association emphasize multidisciplinary teams, including child psychologists for consent.
For transplant biopsies, protocol biopsies—at 3, 6, or 12 months post-transplant—screen subclinical rejection, even in asymptomatic patients. These use smaller needles (16-18 gauge) to preserve graft function. In living-donor evaluations, pre-transplant biopsies assess subclinical disease, influencing donor selection amid ethical debates on “healthy” kidney harvesting.
Intra-procedural monitoring has advanced with point-of-care ultrasound for immediate hematoma detection and Doppler to avoid vascular structures, cutting AVF incidence by 30%. Post-biopsy, 24-hour urine collections quantify hematuria, and MRI or CT angiography diagnoses rare pseudoaneurysms.
Renal biopsy’s utility shines in heterogeneous diseases. In systemic lupus erythematosus (SLE), it classifies lupus nephritis into six ISN/RPS classes, from minimal mesangial (Class I) to advanced sclerosing (Class VI). Class III/IV proliferative forms, showing endocapillary proliferation and “full-house” immunofluorescence (IgG, IgA, IgM, C3, C1q), guide aggressive therapy like mycophenolate mofetil. Serial biopsies track response, with remission defined by <25% activity index reduction.
Diabetic nephropathy, the leading CKD cause, typically spares biopsy due to classic triad (retinopathy, neuropathy, albuminuria). However, atypical presentations—like rapid progression without retinopathy—warrant sampling to rule out superimposed pathologies (e.g., membranoproliferative glomerulonephritis). Biopsy reveals nodular Kimmelstiel-Wilson lesions on LM and thickened glomerular basement membranes on EM, staging via the Renal Pathology Society classification to predict end-stage renal disease (ESRD) risk.
In vasculitis, such as ANCA-associated (e.g., granulomatosis with polyangiitis), pauci-immune crescentic glomerulonephritis dominates, with >50% crescents prompting cyclophosphamide and rituximab. Biopsy differentiates from anti-GBM disease, where linear IgG deposits signal urgent plasma exchange.
Pediatric applications include steroid-resistant nephrotic syndrome, where biopsy identifies genetic causes like NPHS2 mutations in FSGS, shifting management from immunosuppression to supportive care. In hemolytic uremic syndrome (HUS), post-infectious vs. atypical forms are distinguished by thrombotic microangiopathy patterns, influencing eculizumab use.
In amyloidosis, Congo red staining confirms apple-green birefringence, typing via mass spectrometry (AL vs. AA) to target chemotherapy or anti-inflammatory agents. Biopsy also evaluates drug-induced nephrotoxicity, such as calcineurin inhibitor toxicity in transplants, showing striped fibrosis.
Renal biopsy embodies the balance between diagnostic yield and procedural risk, profoundly impacting patient outcomes in nephrology. It not only diagnoses but also stages disease, predicts progression, and evaluates treatment response—essential for conditions like CKD, where early intervention can delay dialysis or transplant. As techniques refine and complications decline, its accessibility expands, underscoring its enduring value in unraveling the kidney’s complex pathologies. With ongoing research into safer methods and adjunctive diagnostics, renal biopsy will continue to illuminate the path to better renal health.
Chat With Me